What Does a Small Electronegativity Difference Reveal
Electronegativity is the tendency of an atom to attract electrons. The most electronegative element is fluorine.
5 3 Polarity And Intermolecular Forces Chemistry Libretexts
If atoms bonded together have the same electronegativity the shared electrons will be equally shared.
. What does a small electronegativity difference reveal about the strength of. What does a small electronegativity difference reveal about the strength of a covalent bond If the difference is less than 5 the bond is non-polar and the sharing is equal. In contrast to substitutional solid solutions determined by Hume.
Electronegativity Difference E E E 1-E 2 For example each HO bond in H 2 O has. It can also be used to predict if the resulting molecule will be polar or nonpolar. Never divulgereveal personal information.
Electronegativity is a measure of an atoms ability to attract the shared electrons of a covalent bond to itself. They are so tiny that we cannot even observe them with our naked eye. Polarity arises due to the differences in electronegativity.
Electronegativity values are useful in determining if a bond is to be classified as nonpolar covalent polar covalent or ionic. A small electronegativity difference indicates that the covalent bond is not very polar. The least electronegative or most electropositive element is francium.
How can the octet rule be used to explain the bonding between magnesium and oxygen. The greater the electronegativity difference between two bonded atoms the greater the percent character of the bond. The higher the number the greater the tendency.
Though divulge and reveal are very similar in meaning there is a slight difference. The degree to which an atom attracts electrons in a chemical bond is described by electronegativity. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent.
A lower average electronegativity means that electrons are held more loosely between atoms so that a moderate difference in electronegativity is enough for electron transfer and an ionic bond. 1How many protons and electrons are in a sulfate ion SO42-. If the atoms in a bond have small electronegativity difference then it will be a covalent bond.
Compare the electronegativities of each pair of atoms. I know the answer is 48 protons and 50 electrons but i do not know how. 4 Polar covalent compounds have a large electronegativity difference while nonpolar compounds have a.
Oxygen needs access to two 18441 results page 11 Chemistry. Therefore these two terms are closely related terms. The closer the difference gets to 21 the stronger the polarity gets.
A difference in electronegativity of 17 results in a bond with 51 ionic character. HO colorwhitemThe Oxygen has an electronegativity of 35. Electronegativity is an atoms tendency to attract electrons to itself in a chemical bond.
Polar bonds and polar molecules. The greater the difference between atom electronegativity values the more polar the chemical bond formed between them. ColorwhitemllcolorwhitellThis creates a difference of 13 so that the electrons are colorwhitemllHcolorwhitempulled.
What you should do is look only at the two atoms in a given bond. Electronegativity - the ability of an atom to attract electrons in a covalent bond. The difference in electronegativity is a gauge for the ionic-ness and the covalent-ness of a particular bond.
This tells us that as the difference in electronegativity between any two elements gets incresingly larger than 17 a bond between these two elements is more likely to be ionic. H The difference of 03 is very small. However there is a distinct difference.
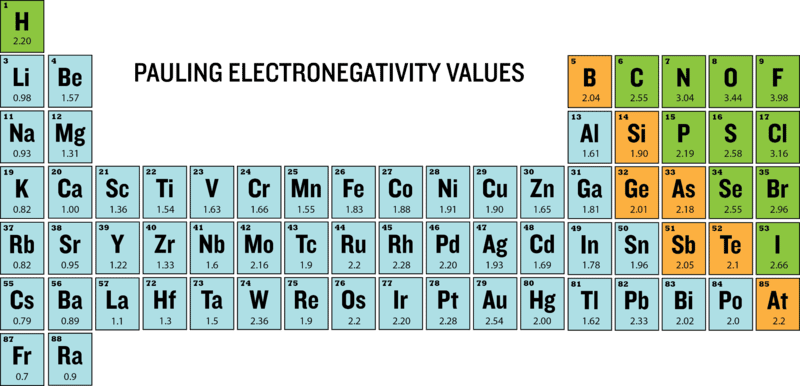
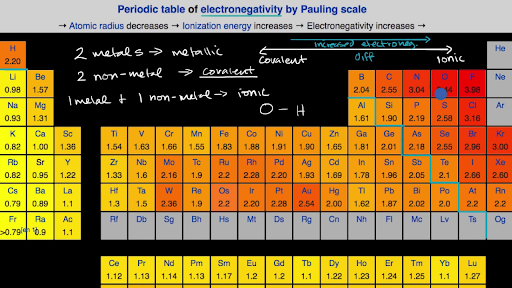
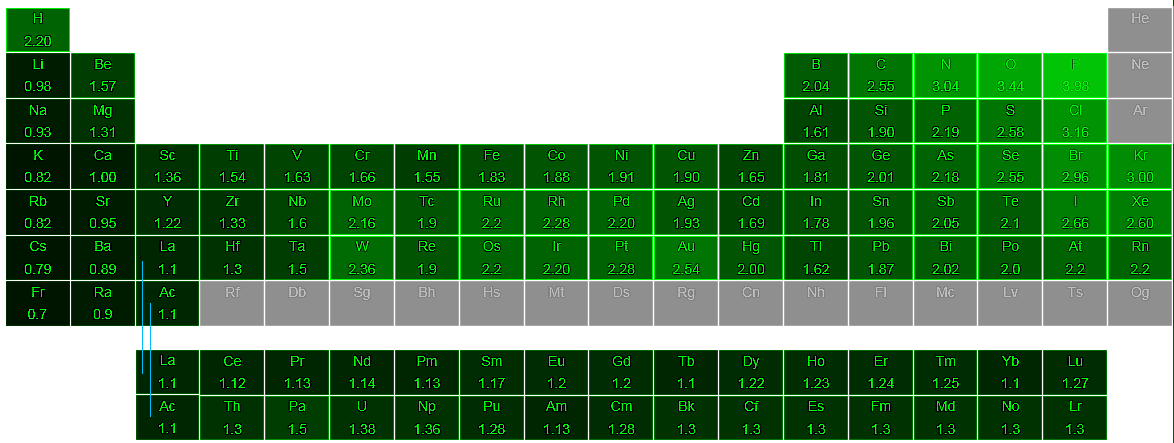
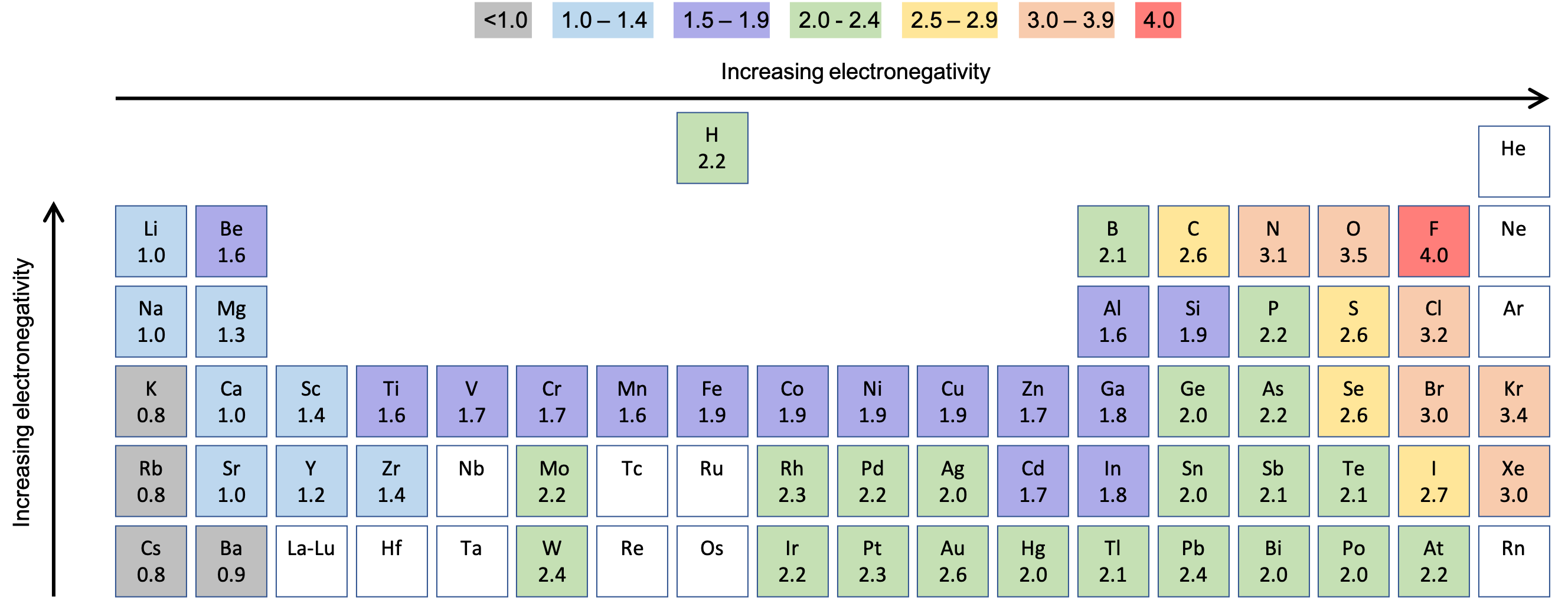
Pauling determined the electronegativity scale based on bond energies between 07 - 4 Fluorine has the highest number of electronegativities. If the electrons of a bond are more attracted to one of the atoms because it is more electronegative the electrons will be unequally shared. The most elctronegative atom is Fluorine and the least electronegative is Francium.
An atom consists of a nucleus. A high average electronegativity means that electrons are being held rather tightly between atoms so that electron-sharing can be maintained even when the difference in. Values for electronegativity run from 0 to 4.
Impurity atoms can be placed in interstitial or substitutional positions and the solubility of impurities is favored when Hume-Romery criteria are satisfied small difference in atomic radii similar crystal structure small difference in electronegativity higher valence dissolves more readily than lower valence. If the difference in electronegativity is significant the bond created is a polar covalent bond such as that formed in water H_2O. The key difference between electronegativity and ionization energy is that electronegativity explains the attraction of electrons while ionization energy refers to the removal of electrons from an atom.
A bond between atoms having a smalllarge difference in electronegativity. Only the absolute difference is important. E E O - E H E 344 - 210 E 144 As the electronegativity difference grows from 000 to 300 the sharing of electrons becomes more skewed.
Both words can be used when referring to information that is secret. A large electronegativity difference leads to an ionic bond. Atoms are the building blocks of all existing substances.
If the difference in electronegativity is greater than 17 the character of the bond will be ionic. The key difference between electronegativity and polarity is that electronegativity is the tendency of an atom to attract the electrons in a bond towards it whereas polarity means the separation of the charges. A small electronegativity difference leads to a polar covalent bond.
Calculate the difference between their electronegativity values. The electronegativity difference between the two elements is 213. If the difference in electronegativity is between 04 and 17 the character of the bond is polar covalent.
If the electronegativity difference between the atoms is large then the bond will ionic in nature.
Definition Of Electronegativity Chemistry Dictionary

9 Trends In The Electronegativity Of Atoms Labxchange

Electronegativity Boundless Chemistry
M8q1 Bonding And Electronegativity Chem 103 104 Resource Book

Lesson Explainer Electronegativity Nagwa

Electronegativity An Overview Sciencedirect Topics

3 Ways To Calculate Electronegativity Wikihow

6 1 Electronegativity And Polarity Chemistry Libretexts

8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts
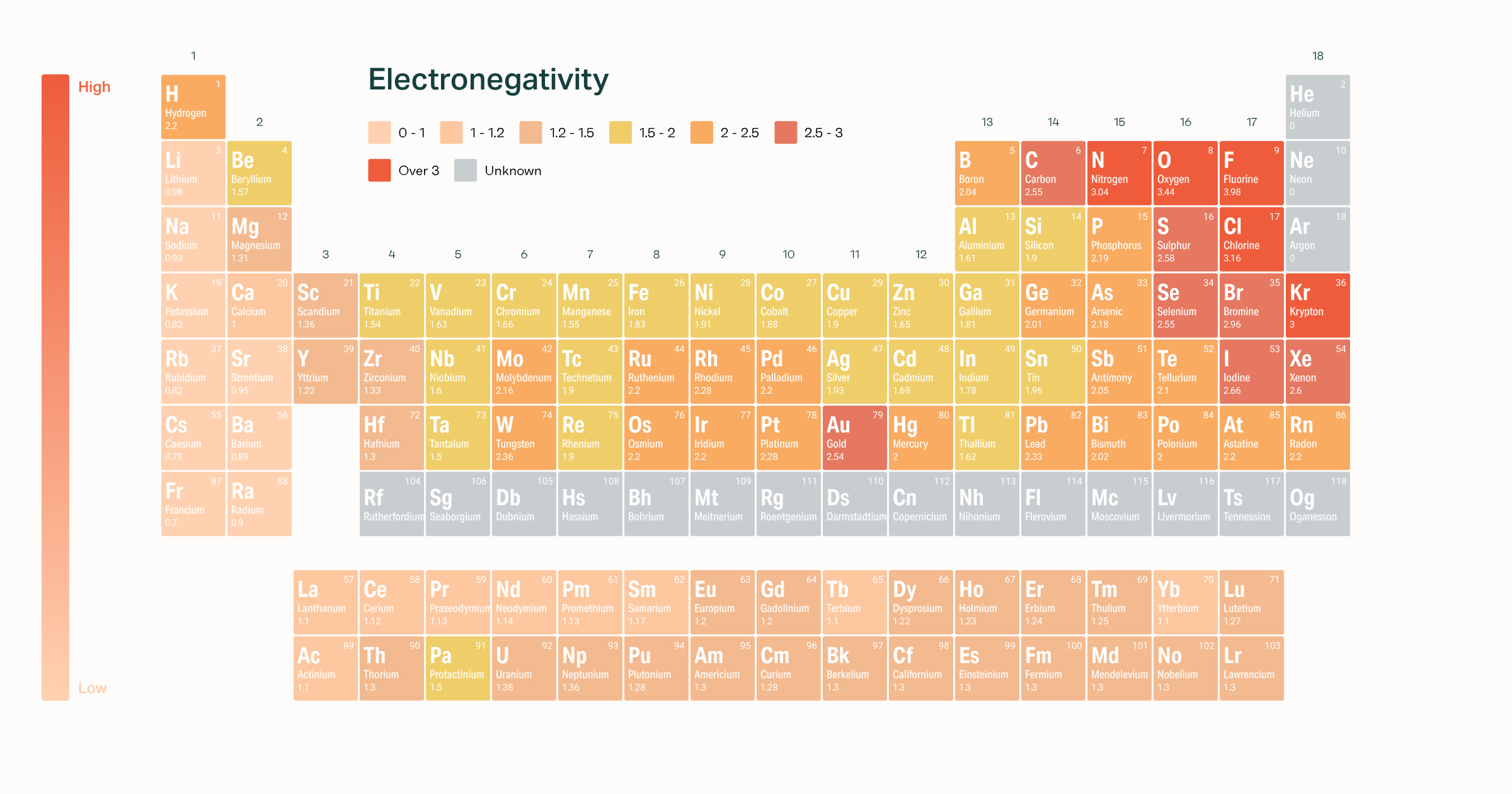
Electronegativity Of The Elements

Electronegativity Definition Value Chart And Trend In Periodic Table

6 4 Polarity Of Molecules Introductory Chemistry
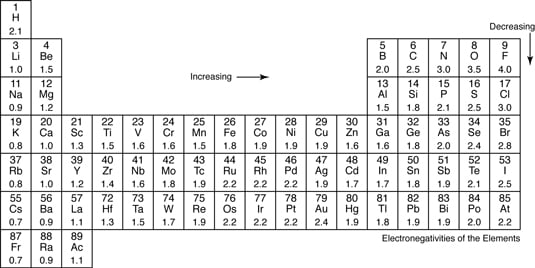
Electronegativity And Polar Covalent Bonding Dummies

Predicting Bond Type Electronegativity Video Khan Academy
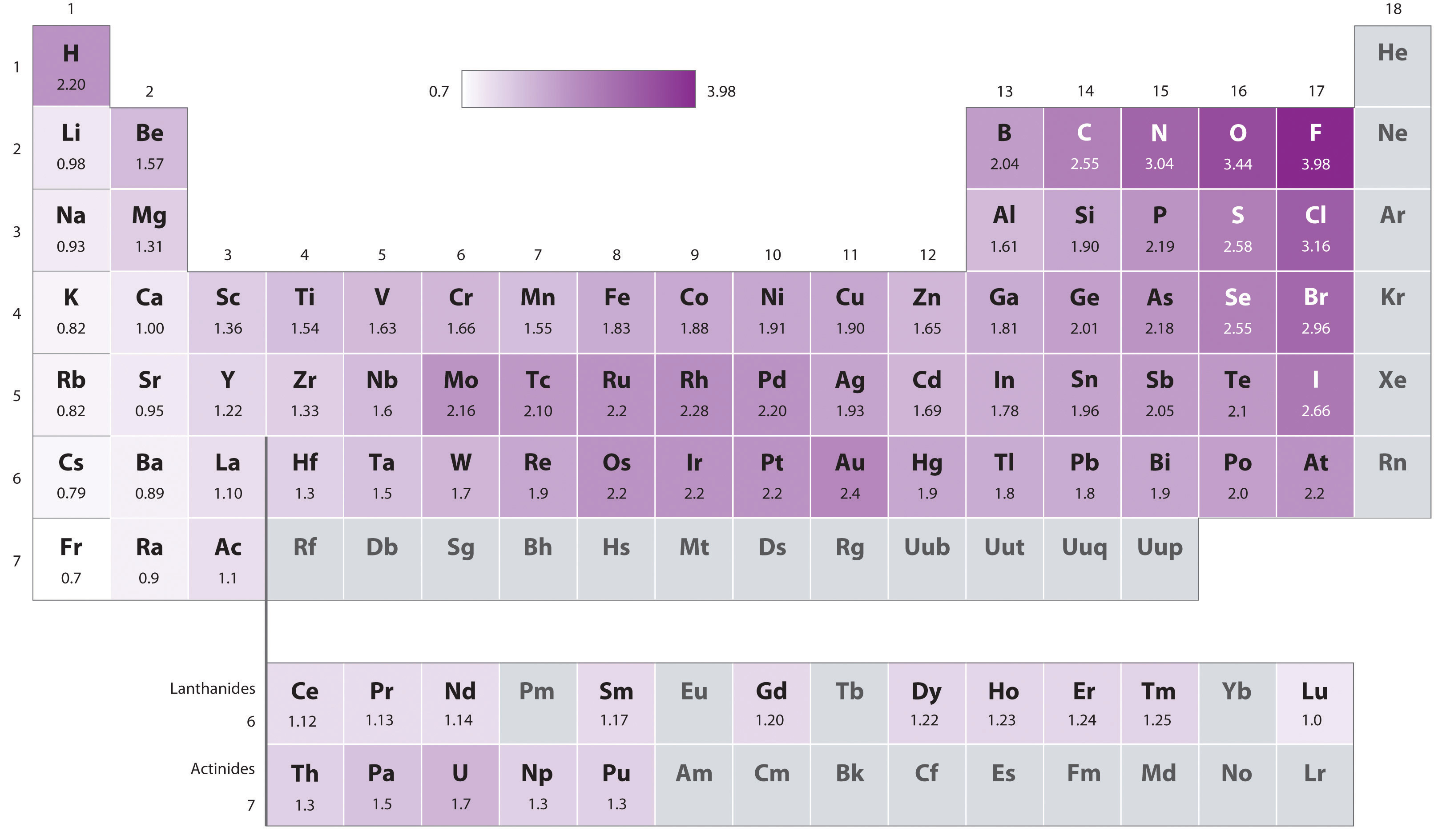
8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts

Electronegativity Bond Scale Surfguppy Chemistry Made Easy Visual Learning Covalent Bonding Chemistry Visual Learning

Electronegativity Definition Value Chart And Trend In Periodic Table

Electronegativity Basic Introduction Periodic Trends Which Element Is More Electronegative Youtube

Periodic Table Of Our Thermochemical Download Scientific Diagram
Comments
Post a Comment